
The coordination principle states that how anions and cations pack together in a crystal structure depends on their relative sizes (radii). in what kind of arrangement) those anions group themselves around a cation is expressed by the c o o r d i n a t i o n p r i n c i p l e. In doing so we can imagine a cation - that is normally relatively small - being in contact with several anions (that are normally larger).

Ionic bonding involves the electrostatic force obtaining between positively charged ions and negatively charged ions (an ion is an electrically charged atom or atomic group).īecause ionic bonds are not directional, we can, as a first approximation, look to the manner in which cations (positively charged ions) and anions (negatively charged ions) bond together in purely geometrical terms, unencumbered by the complexities of aligning orbitals in the specific orientations required for covalent bonds. For example in Ice (H 2O) hydrogen bonding holds water molecules in a hexagonal structure that is reflected in the hexagonal shape of snow flakes. The geometry of the structure is controlled by the shape and charge distribution of the individual molecules and the pattern by which they can be systematically packed together. Molecular crystals are therefore quite soft. The forces that hold the molecules to each other are limited to relatively weak van der Waals and hydrogen bonds. Each molecule in the structure is typically bonded internally with covalent bonds and lacks a residual charge or valence with which to bond with other atoms. Molecular crystals consist of discrete molecules packed together in a systematic way.
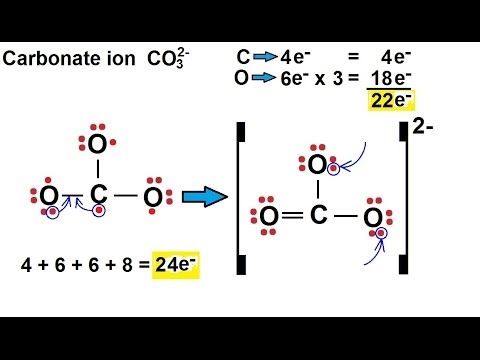
This often prevents the atoms from acquiring a close-packed arrangement. The strong d i r e c t i o n a l nature of covalent bonds requires that the atoms be placed in specific o r i e n t a t i o n s so that orbitals can overlap (See the first part of this website - accessible via back to homepage - The Chemical Bond (Non-classical Series)). In crystals we can encounter several distinct b o n d i n g t y p e s , namely covalent bonding, van der Waals bonding, hydrogen bonding and ionic bonding.Ĭovalent bonds are typically quite strong, so covalent crystals are hard and have high melting points. Deriving the Complex Motif of Calcite (CaCO 3) Chemical bonding and Structure Control in Crystalsīefore we're going to analyze the structure of Calcite crystals in order to find the Complex Motif (that lies at the base of the crystal's tectological structural aspect), something must be said with respect to chemical bonding and ionic radii, both controlling the internal structure of crystals. REMARK : When the reader feels satisfied with our established theoretical foundation (done in previous documents) that allows (single, non-twinned) Crystals to be promorphologically assessed, he or she can skip all the following, and directly proceed with The Promorphology of Crystals. to make the number of charges the same, we need one Ca 2+ ion and two OH - ionsĭeduce the formula for lead(II) sulfate.Promorphology of Crystals Preparation 3-D IV Stereometric Basic forms of Inorganic Beings Promorphology of Crystals.this is two positive charges and one negative charge.If the formula of an ionic compound needs more than one polyatomic ion, the formula of this ion is written inside brackets.Ĭalcium hydroxide contains Ca 2+ and OH - ions: Polyatomic ions are formed from groups of two or more atoms. to make the number of charges the same, we need two Al 3+ ions and three O 2- ions.this is three positive charges and two negative charges.this is two positive charges and two negative chargesĪluminium oxide contains Al 3+ and O 2- ions:.Magnesium oxide contains Mg2 + and O2 - ions: the number of charges are already the same.this is one positive charge and one negative charge.Sodium chloride contains Na + and Cl - ions: The formula for an ionic compound must give the same number of positive and negative charges. Positive ionsĪlthough ionic compounds contain electrically charged ions, they are neutral overall. Similarly, the non-metal elements in groups 6 (IUPAC group 16) and 7 (IUPAC group 17), the ionic charge can be deduced by working out how many electrons must be gained to fill the outer shell. Remember that for the metal elements in groups 1, 2, and 3 the charge on the ion can be deduced by how many outer shell electrons there were in the neutral atom. The formula of an ionic compound can be deduced from the formulae of its ions. They almost always contain at least one metal element and at least one non-metal element. Ionic compounds are made up of oppositely charged ions joined together by ionic bonds.


 0 kommentar(er)
0 kommentar(er)
